The short answer to this question is that the most important features of a laboratory information management system (LIMS) are the features that are most important for your lab. Many labs have similarities, but every lab has its own unique characteristics. You may have heard about a LIMS but don’t know what it can offer. Or perhaps you know what it is broadly but don’t understand in what ways it can help the average laboratory accomplish internal and external stakeholder goals and requirements. These knowledge gaps may be compounded by industry-specific focuses or LIMS vendors who attempt to cater to all industries at once. However, there is an underlying set of functionality that remains consistent across most LIMS, regardless of industry or workflow. This functionality is driven by the underlying purpose of a LIMS, helping labs produce more timely, accurate, and repeatable results more efficiently while also addressing regulatory, standard, management, and economic pressures.
Important LIMS features: The fundamentals
A LIMS can be purpose-built to a specific industry or designed to broadly meet the base needs of most any lab. As such, given different vendor approaches and different industry needs, functionality may vary among LIMS, sometimes significantly. But what are the most important features of these systems?
To be fair, asking a question about “most important features” makes it tough to come up with something other than a subjective answer. On one hand, important to who? The laboratory seeking a LIMS is of course going to have its own workflows and other unique requirements, and that lab will want a LIMS that can address those needs and requirements. On the other, some iteration of the LIMS has been around for decades, and while the way laboratory work has been conducted has changed over time, some LIMS requirements have remained the same. Dating back to the 1970s, LIMS has had the automation of laboratory workflows, reporting, and data management at its core[1], and that is no different today. The birth of LIMS in the 1970s into the early 1980s saw focus placed upon seven areas of need[1]:
- sample management,
- interfacing and networking (i.e., integration),
- data processing and management,
- inventory management,
- document management,
- quality control, and
- reporting.
If one looks carefully at that list of LIMS functionalities, they will notice that those needs essentially follow the sample or specimen’s path throughout the lab, from initial acquisition to results reporting and disposal. The sample is registered in the LIMS system, and connected instruments can analyze the sample and send results back to the LIMS. The resulting data can be processed, managed, and tracked, as can any aliquots and reagents created and used throughout the LIMS workflow. Documents detailing procedures, standardized methods, quality processes, and more can be accessed as needed, and quality checks can be made automatically or by the analyst at the appropriate stages of the workflow, before results are finalized and reported upon. These same needs, and then some, drive LIMS adoption today.[2] As such, most any well-designed LIMS today will have functionality to aid labs with those specific seven needs.
Important LIMS features: The rest of the story
Aside from (and inclusive of) the seven areas of need consistently associated with LIMS over the years, there are other areas along a laboratory’s workflow where LIMS has critically stepped in. Again, these areas may differ from industry to industry; that said, commonalities among industries and lab type remain. This commonality is best reflected in efforts that examine the regulations, standards, guidelines, and best practices affecting laboratories and LIMS today. When examined holistically, we find certain LIMS functionality being driven, at least in part, by those regulations, standards, guidelines, and best practices. This includes[2][3][4][5]:
- audit trails and chain of custody;
- version control;
- electronic signature support;
- barcode, RFID, and label support;
- results review and verification;
- user qualification, performance, and training management;
- instrument and equipment calibration and maintenance management;
- sampling and test method validation;
- statistical trending and control charting;
- nonconformance management;
- corrective and preventive action management;
- backup, retrieval, and retention of data and information;
- robust (i.e., granular) access control and other security management; and
- system validation.
Not necessarily driven by regulations and standards are a few other aspects of a LIMS that can arguably be considered important, including[6][7][8][9]:
- workflow management and automation;
- entity management (e.g., supplier, customer, patient, participant, group, aliquot, cell line, etc.);
- data import and export;
- data analysis, visualization, and dashboarding;
- project and experiment management; and
- robust query tools.
This LIMS functionality and more is put to use not only because of regulatory pressures on laboratories but also management, industry, and economic pressures and goals. As industry veteran Joe Liscouski noted in 2023, when examining the different types of labs, these pressures and goals look roughly similar. Analytical labs have a strong focus on working “efficiently and effectively to produce high-quality and accurate analytical test results while maintaining profitability”; research labs are developing or improving new products “while ensuring at the same time that analyses and studies are conducted consistently, accurately, and ethically”; and quality labs are in the end helping to promote “higher levels of quality, consumer safety, and consumer satisfaction.”[10] In all cases, producing timely, high-quality results that benefit a wide variety of stakeholders while doing so efficiently is critical. This means automating workflows while ensuring timely insights and results that are accurate and demonstrably verifiable as meeting stakeholder requirements, all of which a LIMS can help labs better accomplish.[10][11][12]
Conclusion
The most important thing to remember when considering LIMS is that the needs of your lab now may not be the same a year or five years from now. Understanding what a LIMS can do is the first part of the process. Understanding what would be involved in the LIMS whenever you want or need to make any adjustments, additions of tests, workflows, whole new departments, or even new lab locations, or perhaps pivot in brand new directions, is equally as important. A LIMS is a significant investment, every bit as significant as any instrument. Analysis alone is worthless without the ability to keep track of samples and their statuses, ensure accuracy, and generate professional reports – all in an efficient, cost-effective fashion.
When considering the purchase of laboratory software like LIMS, it is best to involve all relevant stakeholders—including laboratory staff, management, IT, and compliance teams—in the selection process. Gather their input to confirm the chosen LIMS meets everyone’s needs. Determine your budget range for acquiring and implementing a LIMS. Consider the initial costs and ongoing maintenance, support, and potential scalability needs within your financial capabilities.
A LIMS fills many different needs for laboratories, with those acquiring laboratories trying to find the best match given their unique workflows, data management requirements, and budget. However, despite any diversity in functionality based on industry or purpose, a base set of underlying functionality has existed since the LIMS was envisioned in the late 1970s. Sample management, interfacing, data management, inventory management, document management, quality control, and reporting needs originally drove and today still drive LIMS development and use, all of which can be placed within the framework of following a sample or specimen from workflow entry to workflow exit. Additional needs met by a LIMS are often driven in part by regulations, standards, guidelines, and best practices that apply to the laboratory. Here a LIMS steps in by providing audit trails, electronic signatures, method validation, nonconformance management, and granular access controls, among other functionality. However, some important LIMS functionality is also driven not by regulations and standards but rather economic and stakeholder pressures. This includes workflow management and automation tools, entity management, dashboards, experiment management, and query tools, among others. All these LIMS functions and more help labs better achieve their goals.
The LabLynx LIMS Solution
The ELab LIMS can also be easily configured through the user interface (UI) by your authorized users, and also customized through backend development if required, by our own experienced, professional developers, to meet your lab’s specific needs.
Below are a few key features provided in the LabLynx ELab LIMS:
- Sample tracking and management
- Sample and result batching
- Data warehouse – storage, management, access
- Workflow management – Create/modify process steps, sequential processing paths, etc.
- Test management – Create/modify tests, parameters, limit sets, specifications, etc.
- Instrument integration
- Internal and external file or data upload/linking
- QA/QC functionality and Control Charting
- Data and trend analysis
- Custom reporting and barcode support
- User-level roles for security management and user-based configurations
- Extensive flexibility through user-configuration (low-code/no-code)
- Audit trail and chain of custody (COC)
- Document/files creation and management with ISO-compliant versioning
- Standards/regulatory compliance – ISO, 21 CFR, EPA, HIPAA, FDA, GmP
- Easily add additional departments and locations
- Operating system and browser agnostic: accessible via any browser on any device
- Third-party software integration
- Hosting – cloud, on premises, or hybrid
- Task and event scheduling
- Query/search capabilities – multiple criteria
- Inventory management
- Instrument calibration and maintenance management
- Training, support, and additional services like validation documentation, through LiMShelp
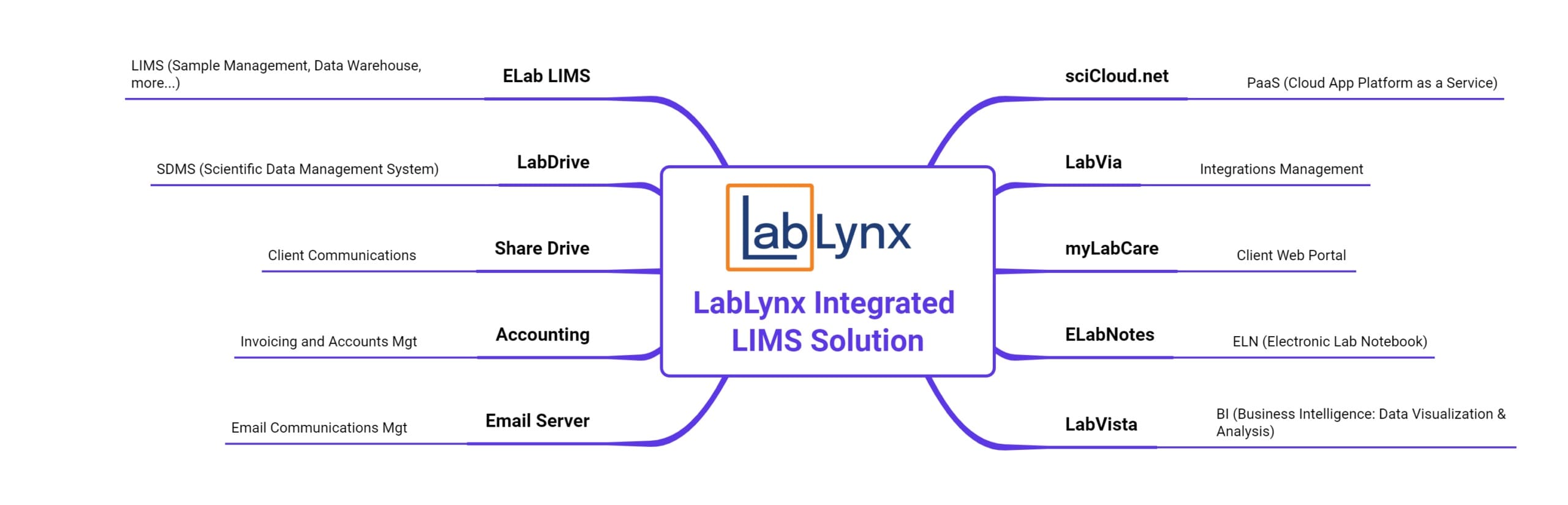
Additionally, LabLynx is unique in offering a LIMS suite of platforms and applications that are designed to work together seamlessly with each other and with your existing infrastructure to provide a complete data management solution for your lab or labs. These include:
- ELab Notes Electronic Lab Notebook (ELN)
- LabDrive Scientific Data Management System (SDMS)
- LabVia Integrations Manager
- LabVista Business Intelligence (BI) tool for data analytics and visualization
- SciCloud.net® Cloud platform (platform as a service, or PaaS)
That is what the LabLynx LIMS solution gives you: not simply a LIMS, but a complete data management ecosystem – or the elements you need to complete your existing overall data management ecosystem. Contact us to set up a time to discuss your lab’s data management needs!
References
- Gibbon, G.A. (1996). “A brief history of LIMS”. Laboratory Automation and Information Management 32 (1): 1–5. doi:10.1016/1381-141X(95)00024-K. http://dx.doi.org/10.1016%2F1381-141X%2895%2900024-K
- Kranjc, Tilen (16 August 2021), Zupancic, Klemen; Pavlek, Tea; Erjavec, Jana, eds., “Introduction to Laboratory Software Solutions and Differences Between Them” (in en), Digital Transformation of the Laboratory (Wiley): 75–84, doi:10.1002/9783527825042.ch3, ISBN 978-3-527-34719-3. https://onlinelibrary.wiley.com/doi/10.1002/9783527825042.ch3
- Douglas, S.E. (December 2022). “LII:LIMSpec 2022 R2”. LIMSwiki. Retrieved 20 March 2024. https://www.limswiki.org/index.php/LII:LIMSpec_2022_R2
- Douglas, S.E. (January 2023). “LIMS Q&A:What are the key elements of a LIMS to better comply with ISO/IEC 17025?”. LIMSwiki. Retrieved 20 March 2024. https://www.limswiki.org/index.php/LIMS_Q%26A:What_are_the_key_elements_of_a_LIMS_to_better_comply_with_ISO/IEC_17025%3F
- Berezin, Casey-Tyler; Aguilera, Luis U.; Billerbeck, Sonja; Bourne, Philip E.; Densmore, Douglas; Freemont, Paul; Gorochowski, Thomas E.; Hernandez, Sarah I. et al. (7 December 2023). Markel, Scott. ed. “Ten simple rules for managing laboratory information” (in en). PLOS Computational Biology 19 (12): e1011652. doi:10.1371/journal.pcbi.1011652. ISSN 1553-7358. PMC PMC10703290. PMID 38060459. https://dx.plos.org/10.1371/journal.pcbi.1011652
- Nelson, Elizabeth K; Piehler, Britt; Eckels, Josh; Rauch, Adam; Bellew, Matthew; Hussey, Peter; Ramsay, Sarah; Nathe, Cory et al. (1 December 2011). “LabKey Server: An open source platform for scientific data integration, analysis and collaboration” (in en). BMC Bioinformatics 12 (1): 71. doi:10.1186/1471-2105-12-71. ISSN 1471-2105. PMC PMC3062597. PMID 21385461. https://bmcbioinformatics.biomedcentral.com/articles/10.1186/1471-2105-12-71
- “ASTM E1578-18 Standard Guide for Laboratory Informatics”. ASTM International. 23 August 2019. Retrieved 20 March 2024. https://www.astm.org/e1578-18.html
- Grand, Alberto; Geda, Emanuele; Mignone, Andrea; Bertotti, Andrea; Fiori, Alessandro (1 January 2019). “One tool to find them all: a case of data integration and querying in a distributed LIMS platform” (in en). Database 2019. doi:10.1093/database/baz004. ISSN 1758-0463. PMC PMC6352757. PMID 30698777. https://academic.oup.com/database/article/doi/10.1093/database/baz004/5304001
- Cooper, David R.; Grabowski, Marek; Zimmerman, Matthew D.; Porebski, Przemyslaw J.; Shabalin, Ivan G.; Woinska, Magdalena; Domagalski, Marcin J.; Zheng, Heping et al.. (2021), Chen, Yu Wai; Yiu, Chin-Pang Bennu, eds., “State-of-the-Art Data Management: Improving the Reproducibility, Consistency, and Traceability of Structural Biology and in Vitro Biochemical Experiments” (in en), Structural Genomics (New York, NY: Springer US) 2199: 209–236, doi:10.1007/978-1-0716-0892-0_13, ISBN 978-1-0716-0891-3, PMC PMC8019398, PMID 33125653. Retrieved 2024-03-20. http://link.springer.com/10.1007/978-1-0716-0892-0_13
- Liscouski, J. (July 2023). “1. Introduction to LIMS and its acquisition and deployment”. In Douglas, S.E.. Justifying LIMS Acquisition and Deployment within Your Organization. LIMSwiki. Retrieved 20 March 2024. https://www.limswiki.org/index.php/LII:Justifying_LIMS_Acquisition_and_Deployment_within_Your_Organization/Introduction_to_LIMS_and_its_acquisition_and_deployment
- “2022 Laboratory Informatics: Progress Snapshot on Enabling the Digital Lab of the Future” (PDF). Astrix Technology, LLC. June 2022. pp. 18–23. Retrieved 20 March 2024. https://astrixinc.com/wp-content/uploads/2022/06/Progress-Snapshot-on-Enabling-the-Digital-Lab-of-the-Future-v4a.pdf
- Douglas, S.E. (December 2023). “LIMS Q&A:What are the organizational justifications for a laboratory information management system (LIMS)?”. LIMSwiki. Retrieved 20 March 2024. https://www.limswiki.org/index.php/LIMS_Q%26A:What_are_the_organizational_justifications_for_a_laboratory_information_management_system_(LIMS)%3F